ABOUT CYTIVA
With a rich heritage dating back hundreds of years, Cytiva brings a wealth of technical expertise and talent, a broad and deep portfolio, and exceptional service help researchers and biopharma advance therapeutics at every stage from discovery to delivery.
We supply the tools and support our customers need to work better, faster, and safer, leading to the delivery of transformative medicines to patients. Our combined portfolio includes well-recognized names such as Allegro™, Supor™, iCELLis™, and Kleenpak™, in addition to ÄKTA™, Amersham™, Biacore™, FlexFactory™, HyClone™, MabSelect™, Sefia™, Whatman™, and Xcellerex™. Visit cytiva.com to learn more.
PRODUCT VIDEOS
FEATURED PRODUCTS
-
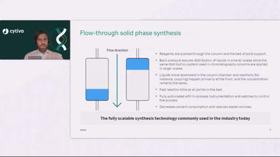
Explore how smart column design supports consistent, high‑yield oligonucleotide synthesis, which formats fit development, and how optimized features help improve efficiency and safety.
-
Discover a system with a flexible synthesis scale, intuitive software, and robust process control that is ideal for labs moving from research to production.
-
Research Use Only (RUO) lipid nanoparticle (LNP) reagents to deliver RNA into hematopoietic stem cells (HSCs) for cell and gene therapy applications.
-
Off-the-shelf, research use only lipid nanoparticle (LNP) reagents to deliver RNA into T-cells.
-
Bring confidence to filtration with an automated single‑use filtration system for pilot‑ and small‑scale manufacturing. This system is intended for crossflow filtration applications in both upstream and downstream workflows.
-
Learn about a scalable, single-use platform with built-in automation and regulatory compliance that enables standardized manufacturing workflows for mRNA-LNP drug product production.
-
The NanoAssemblr® commercial formulation system is an automated, single-use system for the clinical and commercial production of lipid nanoparticles (LNPs) under cGMP conditions. Designed for efficient changeover and robust manufacturing processes, the system enables operational flexibility and standardized manufacturing of genomic medicines.
-
Discover a one-stop-shop for LNP technologies that provides access to expertise in formulation and analytics for successful outcomes for payload and target applications. Explore end-to-end biopharma services and systems.
-
Designed for small-scale manufacturing, this single-use liquid chromatography system offers the performance and documentation required for a GMP-regulated environment.
-
Learn about coated magnetic particles that easily isolate and extract mRNA from a variety of sources and enable you to perform RT-PCR cDNA library construction, affinity purification, and more.
-
Synthesize oligonucleotides at scales of 10–100 mmol for early to mid-phase clinical trials or for diagnostic kits.
-
Looking for a user-friendly and compact oligonucleotide synthesizer with a large scale range? Discover a pilot-scale system that will take your drug through clinical development.
-
Explore systems for automated oligonucleotide synthesis, offered at a range of scales to support easy process development, optimization, scale up and transfer. Find the system that matches your needs.
-
The rise in approved oligo therapeutics has led to increased pressure on oligo manufacturers. Learn about modern chromatography resins, how they can help increase productivity in manufacturing, and more.
-
Discover the platform for oligonucleotide development and scale-up that is the result of 30 years of collaboration with leading oligonucleotide pharma. Explore this solution's benefits, specifications, and more.
-
OligoProcess covers a range of 10 to 1800 mmol and is suitable for quantities required in clinical phases and commercial production.
-
By combining disruptive technology platforms with unparalleled genomic medicine development expertise, we're positioned to accelerate the development of LNP formulations and drug products.
-
Ignite simplifies the transition into clinical programs by incorporating these fundamental process steps for scale up in the earliest stages of pre-clinical development.
-
GenVoy-ILM™ T Cell Kit for mRNA, Ignite is an lipid nanoparticle (LNP) reagent mix optimized for the delivery of messenger RNA (mRNA) or Cas9 mRNA/sgRNA into activated human primary T cells.
-
With NxGen™ technology on the NanoAssemblr® Blaze™, important studies can be conducted efficiently with a process that mirrors a clinical scale implementation.
WEBINARS AND PODCASTS
APPLICATION NOTES
- mRNA Manufacturing With Fed-Batch In Vitro Transcription
- Oligonucleotide Purification And Synthesis
- How To Successfully Isolate Your T Cells
- TFF Cassettes With 100 kDa Membranes For RNA And LNP Applications
- Single‑Use Filtration System For Pilot‑ And Small‑Scale Manufacturing
- Exosome Isolation By TFF And Size Exclusion Chromatography
- Standardize Scale-Up And Reduce Time To Market With Mixing Cartridges
- Single-Use Liquid Chromatography System
- Coated Magnetic Particles For RNA Preparation
- Compact Oligonucleotide Synthesizer
CONTACT INFORMATION
Cytiva
100 Results Way
Marlborough, MA 08855-1327
UNITED STATES
Phone: 800-526-3593
FEATURED ARTICLES
-
Learn how buffer choice, gradient strategy, and pretreatment steps influence recovery, purity, and resolution when purifying a native RNA oligo, with analytical confirmation and practical guidance.
-
Explore how an optimized solid‑support design enables higher synthesis scales, steadier pressures, and lower solvent use while preserving yield and purity across diverse oligonucleotide lengths.
-
Lipid nanoparticles enable precise, non-viral gene editing in T cells to achieve high HDR efficiency and viability in scalable workflows. Explore how this approach overcomes viral vector limitations.
-
Get a clear view of oligonucleotide development from synthesis to purification and filtration with practical insights to help you streamline workflows and stay ahead in the fight against disease.
-
Explore how lipid nanoparticles are revolutionizing cell therapy workflows to enable the precise delivery of mRNA and gene editing tools for next-generation treatments like CAR T and HSC therapies.
-
Lipid nanoparticles enable efficient gene editing in hard-to-transfect cells like T-cells and blood stem cells, which offers scalable, clinically relevant methods for developing advanced therapies for cancer and rare diseases.
-
Lipid nanoparticles have revolutionized drug delivery, ushering in a new era of nanomedicine. Discover how advanced analytical tools are accelerating next-generation LNP therapeutics.
-
Gene therapies are expanding beyond rare diseases, but high costs hinder access. Discover how innovations in viral vector manufacturing could make these breakthroughs more affordable.
-
Cell therapies, using reprogrammed immune cells, offer innovative treatments for diseases. Discover how lipid nanoparticles (LNPs) enhance these therapies by delivering RNA for gene modulation and next-gen treatments.
-
Discover how self-replicating RNA (srRNA) can revolutionize vaccine development with sustained protein expression, lower doses, and fewer side effects to advance next-gen RNA therapeutics.