What Does FDA's Draft Platform Guidance Mean For CGT Companies?
By Kimberly Benton, Dark Horse Consulting Group

The U.S. FDA released the much-anticipated draft guidance Platform Technology Designation Program for Drug Development1 on May 28, 2024. This guidance was issued to establish a program for designation of platform technologies as required by the PREVENT Pandemics Act2 and provides information on how FDA intends to implement the program. Comments may be submitted to the public docket3 until July 29, 2024.
When federal statutes and FDA guidances introduce new designations and programs, a slate of questions come to mind for product developers, including: What is this? Is my product/technology eligible? How would this benefit me? How do I get this designation? Let’s consider these questions from the perspective of cell and gene therapy products and technologies used in their manufacture.
What Is A Platform Technology?
The draft guidance defaults to the statute’s language2 for the definition of platform technology:
“A platform technology is a well understood and reproducible technology, which may include a nucleic acid sequence, molecular structure, mechanism of action, delivery method, vector, or a combination of any such technologies that FDA determines to be appropriate, where it:
- is incorporated in or utilized by a drug and is essential to the structure or function of such drug;
- can be adapted for, incorporated into, or utilized by, more than one drug sharing common structural elements; and
- facilitates the manufacture or development of more than one drug through a standardized production or manufacturing process or processes.”
The term drug in the definition also includes biologics. This definition could apply to many types of technologies used in cell and gene therapies. The guidance gives the example of lipid nanoparticles (LNPs) for gene therapy and includes a footnote that other types of cell and gene therapy products may be eligible. FDA has raised other examples in public presentations in recent years, including adeno-associated virus (AAV) vector backbones with different transgenes and CRISPR-Cas9 gene editing using different guide RNAs.
Is My Product/Technology Eligible For The Platform Designation Program?
To be considered eligible for the platform designation program a product/technology meeting the platform technology definition must also meet the following criteria:
- incorporated in, or used by, an approved drug or biological product;
- preliminary evidence demonstrates that the platform technology has the potential to be incorporated in, or used by, more than one drug without an adverse effect on quality, manufacturing, or safety; and
- the platform technology has a reasonable likelihood to bring significant efficiencies to drug development or manufacturing and to the FDA review process.
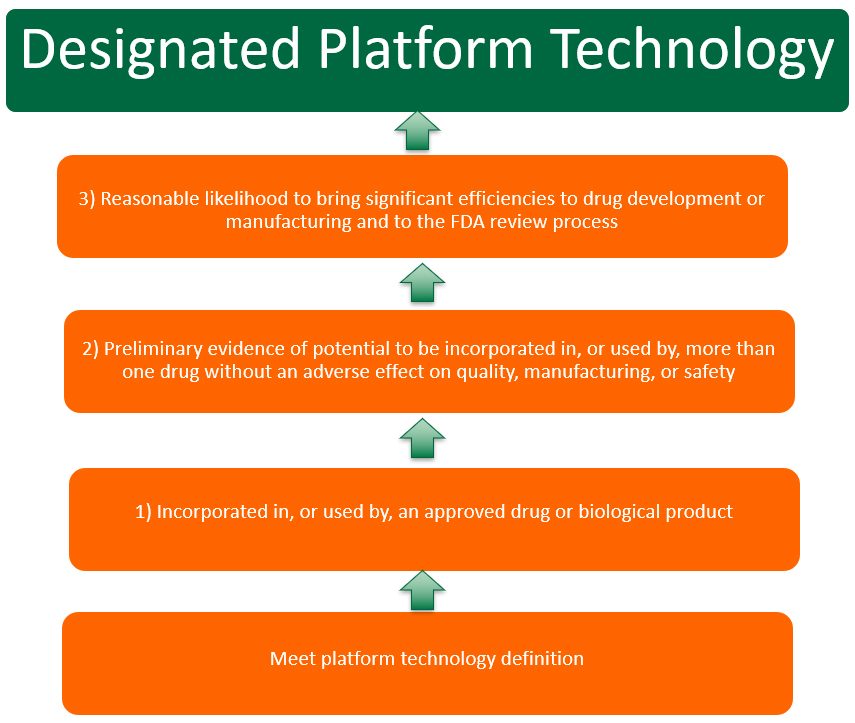
The source of these eligibility criteria is the federal statute2, and FDA is merely reiterating them in the draft guidance. The limitation of eligibility to technologies used in at least one approved BLA or NDA significantly narrows the number of entities that can seek designation. Only those with legal right of reference to the data on the platform technology in an approved application are eligible to seek platform technology designation. The right to reference data must be documented with typical letters of cross-reference.
Preliminary evidence mentioned in the second criterion is also defined in the statute.2 The last part of the preliminary evidence definition is most helpful:
findings from tests or studies that evaluate proposed use of the platform technology in an already approved product, evaluate the proposed use of the same platform technology in a new drug product, or draw comparisons between the use of a platform technology across scenarios.
The third criterion may seem straightforward, but the draft guidance addresses how this could be a make-or-break criterion for designation. Specifically, FDA states that technologies for which current review processes already reflect the use of the well understood technology or there is a public standard may be considered inappropriate for designation. Compared to traditional biologics and drugs, the relative novelty of cell and gene therapy products may increase the likelihood that FDA will consider most candidates for platform technology to meet the criterion of bringing significant efficiencies. However, with six approvals in the U.S., would FDA consider lenti-based CAR T cells to meet the criterion of bringing significant efficiencies? Sponsors should present a strong justification in a designation request.
How Would Platform Technology Designation Benefit Me?
The benefits of platform technology designation are the potential to leverage data from the approved product to create efficiencies in the development of the new product(s) and the potential for additional interactions with the FDA to discuss such leveraging. Areas for leveraging might include referencing nonclinical data from the approved product to reduce or eliminate the need for nonclinical studies of the new product. Product manufacturing leveraging may include use of data from the approved product to establish safety, purity, potency, and quality of the new product. Additional considerations may be whether process performance qualification, process validation, and stability data from the approved product may be leveraged for the new product. The ability to rely on previously obtained data from the approved product should speed the development of the new product and result in a faster path to BLA submission and approval.
The potential to facilitate efficiencies during the IND and BLA review stage is another possible benefit. The ability to reference data previously reviewed reduces the FDA’s review burden, which, in turn, can reduce the burden for an IND or BLA sponsor with fewer information requests. It also can reduce the risk of IND hold or BLA refuse-to-file or complete response. During BLA review, facility inspection may be waived or the risk of significant objectionable observations may be reduced. These efficiencies may add up to faster time to BLA submission and potentially a faster completion of FDA review of the BLA.
How Do I Request A Platform Technology Designation?
The draft guidance provides information on how to request designation as a platform technology. The main elements of the package to support a request for designation are:
- Explanation of how the technology meets the definition of platform technology as described in the guidance and statute
- Identification of the approved BLA or NDA in which the technology was incorporated
- Comparison of the approved and new product, including the shared structural elements and how the platform technology is used in both
- Scientific justification on how the platform technology will be incorporated into the new product with no or only very minor differences from the approved product and how it maintains the safety and quality profile
- A risk assessment of the differences between the approved and new product, how the differences could affect the use of the platform technology, and the extent to which data from the approved product can be leveraged to support the new product — the sponsor should identify the data intended to be leveraged.
- Position on how the use of the platform technology will bring significant efficiencies to the development, manufacturing process, and FDA review process for the new product
The draft guidance suggests that sponsors can discuss the intent to request platform designation with FDA in a meeting. Sponsors can submit a request for platform designation with the original IND for the new product or at any time after. FDA cautions that a request for platform designation should be made only once adequate supportive information has been obtained to meet the designation criteria. FDA will make a determination on the request for platform technology designation within 90 days of receipt.
Boon For Companies With Approved Products And A Bummer For Everyone Else
This draft guidance has already drawn broad interest. Currently just over two dozen unique companies have an approved cell and gene therapy product in the U.S. Only those companies, and any party to whom they grant right of cross-reference, are eligible to apply for platform technology designation in the cell and gene therapy field. Nothing in the draft guidance indicates that entities in this narrow group need to wait for publication of the final version of the guidance before approaching FDA to discuss their interest in applying for platform technology designation. The existing concept of applying prior knowledge among a company’s products raises questions about the magnitude of benefit that may be effectively realized with platform technology designation program and, therefore, how many in the eligible pool will pursue the designation.
Other players in the cell and gene therapy sector that do not yet have an approved product will likely be unhappy with the limitations of eligibility. The concept of platforms and broader leveraging of knowledge has been a topic of many public discussions in the field in recent years. Many of these discussions have included knowledge leveraging at investigational stages and the hopes it could lead to a reduction in regulatory burden across classes of products. The draft guidance notes that designation of a platform technology does not give third parties additional rights to reference information from an approved product application containing that platform technology if they do not own or have full rights of reference to it. In other words, only approved BLA holders and those to whom they specifically authorize cross-reference rights will benefit from a platform technology designation.
As noted earlier, the public docket3 for the draft guidance is open and comments will be accepted through July 29, 2024. At the time of writing of this article, at least one commenter requested an extension of this comment period. The adherence to the statutory language in the draft guidance suggests that FDA is unlikely to make large revisions in the final version. However, the submission of comments to the docket will make FDA aware of stakeholders’ interests and concerns and potentially lead to FDA to consider other avenues by which they may be addressed.
References:
- Platform Technology Designation Program for Drug Development. Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. 2024
- Section 506K of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 356k) was added by the PREVENT Pandemics Act, which was enacted as part of the Consolidated Appropriations Act, 2023 (Public Law 117-328).
- Platform Technology Designation Program; Draft Guidance for Industry; Availability; Agency Information Collection Activities; Proposed Collection; Comment Request
About The Author:
 Kimberly Benton, Ph.D., served for over 22 years at the U.S. FDA/CBER in regulation of cell and gene therapy products before joining Dark Horse Consulting in January 2023 as master principal and head of regulatory. During her time at FDA/CBER she served as associate director for regulatory management in the Office of Tissues and Advanced Therapies (now the Office of Therapeutic Products), deputy director of the Division of Cellular and Gene Therapies in the Office of Cellular, Tissue, and Gene Therapies, chief of the cell therapies branch, and CMC reviewer of cell therapies.
Kimberly Benton, Ph.D., served for over 22 years at the U.S. FDA/CBER in regulation of cell and gene therapy products before joining Dark Horse Consulting in January 2023 as master principal and head of regulatory. During her time at FDA/CBER she served as associate director for regulatory management in the Office of Tissues and Advanced Therapies (now the Office of Therapeutic Products), deputy director of the Division of Cellular and Gene Therapies in the Office of Cellular, Tissue, and Gene Therapies, chief of the cell therapies branch, and CMC reviewer of cell therapies.
